Hi, dear
How are you?
Let’s see what is difference between Heat and Temperature.
I am sure, after reading this article, your all doubts will be cleared.
First, we will see the difference between Heat and Temperature in tabular form, and then we will discuss it thoroughly.

| HEAT | TEMPERATURE |
| Heat is a form of energy that produces a sensation of hotness and warmness. | Temperature is the measure of the degree of hotness and coldness of the body. |
| Heat is the sum of energies of all molecules in the body. | Temperature is the measure of the average kinetic energy of the molecule in the body. |
| Heat flows from higher temperature to lower temperature. | Temp. is the condition that determines the direction of heat. |
| Heat is the cause | Temperature is the effect |
| Heat is a mode of energy transfer. | Temperature is just a measurement of hotness or coldness. |
SI unit – joule | SI unit – Kelvin |
| Measured by device calorimeter | Measured by Thermometer, hotness meter |
| Represented as ‘Q’ | Represented as ‘T’ |
Table No. 1:Difference between Heat and Temperature
What is HEAT?
Definition: Heat is the sum of energies of all molecules (total energy) in the body or substance.
Here, energy is referred to as the kinetic energy of the molecules. Because we know that when we add heat to a substance( whether it is solid, liquid, or gas) it expands means the kinetic energy of the molecules increases, meaning molecules start vibrating, and they move faster.
And as I mentioned above, Heat is the sum of the kinetic energy of all the molecules.
OR
In simple words, Heat is a form of energy that produces a sensation of hotness and warmness.
Now,
What is TEMPERATURE?
Definition: Temperature is the measure of the average energy (kinetic energy) of the molecular motion in a substance.
OR
Temperature is the measure of the degree of hotness or coldness.
Now we will try to understand heat and temperature with a real-life example-
Example 1: Consider a glass of water containing 1 kg of water. Suppose that 1 kg of water contains 1, 2, 3, 4,………., n molecules. Now we will add heat to the water with the help of a gas burner. When we add heat to the water, we know that the kinetic energy of the molecules will increase.
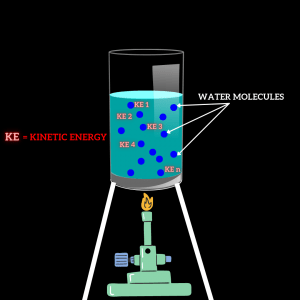
Fig. 1: Apparatus to describe Heat and Temperature
Kinetic Energy is formulated as,
K.E.= ½ mv2
Where,
m= mass of the molecule
v= velocity of the molecule
So, from the above two definitions, we can write,
Heat = KE1+ KE2+ KE3+KE4+…….+KEn
And
Temperature = [ KE1+ KE2+ KE3+KE4+…….+KEn ] / n
Example 2: In the morning when you go for a bath, how do you check your water hotness, by your hand or thermometer?
Yes, by simply your hands. Heat is the sensation of hotness or coldness. But, how you are deciding something is hot or cold.
If you are saying that, whose temperature is more is hot and whose temperature is less is cold, then you are wrong.
Something is hot or cold is depend upon your body temperature.
The human body temperature is about 37 °C. So, water at 30 °C is cold for us but the same water is hot for snakes whose body temperature is about 17.7 to 22.3°C.
So, in the scientific world, we can’t say that something is hot or cold. We need an exact value, that’s why the temperature term is introduced.
Therefore, HEAT is a sensation of hotness or coldness and TEMPERATURE is the measure of hotness or coldness.
Heat is measured by a device called a calorimeter.
James Prescott Joule introduced the unit of heat in 1845.
SI Unit of Heat is – joule(J)
Other units are- calorie (Cal), BTU(British Thermal Unit)
Temperature is measured by a thermometer.
SI Unit of Temperature is- Kelvin (K)
Other units are- Fahrenheit (°F), Celsius (°C)
If you want to know more about Temperature, its units, and what is
absolute zero temperature Click Here
Example 3: Consider two glasses of water containing 1 kg of water each. Then this water is heated up to 90 °C. Then we can say that both glasses have the same temperature and same heat because it has the same no. of molecules of water.
 Fig. 2: Apparatus to show the same quantity of water has the same heat if the temperature is the same
Fig. 2: Apparatus to show the same quantity of water has the same heat if the temperature is the same
Suppose glass A contains 1 kg of water, whereas glass B contains 0.5 kg of water. Then heat is added to both glasses until the thermometer shows a reading of 90 °C.
Now, there is a fun fact. Although both the glasses of water have the same temperature, the heat contained by them is different. Why?
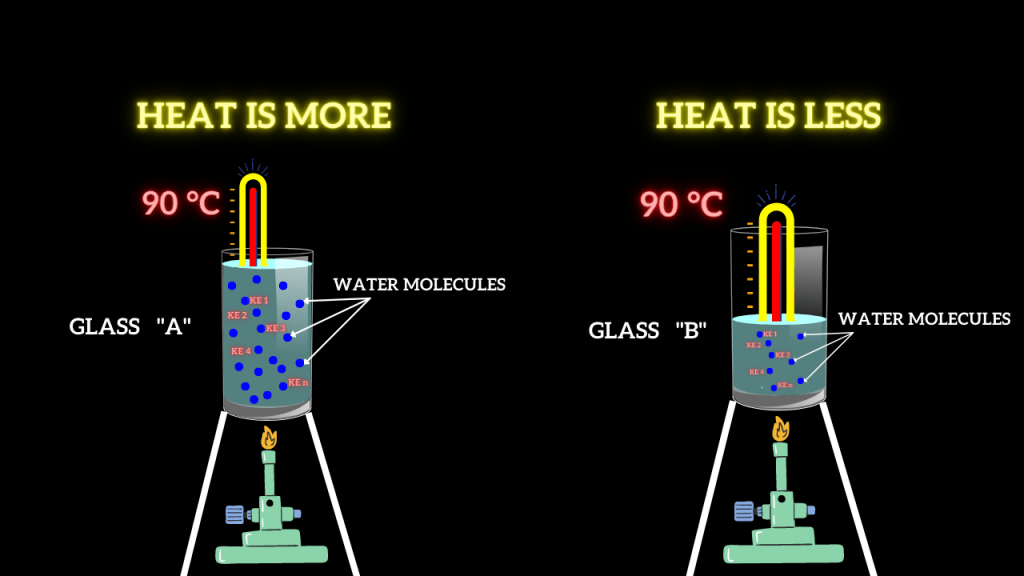 Fig. 2: Apparatus to show the different quantity of water has different heat although the temperature is the same
Fig. 2: Apparatus to show the different quantity of water has different heat although the temperature is the same
Exactly!
Glass A has more heat than glass B because it has more molecules of water than glass B.
So, your morning cup of tea

That’s why ocean water is responsible for winds, hurricanes, and monsoons but not your hot cup of tea.
Isn’t it amazing
A small change in the temperature of the ocean, like only half or 1 °C, tends to massive effect on the environment. Therefore oceans are massive reservoirs of heat on the earth.
That is why scientists are worried about global warming. And our responsibility is to find different ways to reduce it.
SAVE ENERGY, SAVE LIFE
Therefore we can say that heat is dependent upon mass but the temperature is not.
Therefore more heat is required for glass A to raise its temperature up to 90 °C than glass B.
Heat added can be calculated by,
Q = m X cp X Δt
Where,
Q = heat required or added
m= mass of water
cp= specific heat of water at constant pressure
Δt= change in temperature
So, we know that
For glass A
m= 1 kg
cp= 4.182 KJ/kg K or KJ/Kg °C
Δt= in our case we are raising the water temperature from 30 °C to 90 °C
Therefore, Δt= (90-30)°C
For glass B
m= 0.5 kg
cp= 4.182 KJ/kg K or KJ/Kg C
Δt= in our case we are raising the water temperature from 30 °C to 90 °C
Therefore, Δt= (90-30) °C
Therefore,
Q of glass A = 1 X 4.182 X 60 = 250.92 KJ
And
Q of glass A = 0.5 X 4.182 X 60 = 125.46 KJ
Example 4: Second law of thermodynamics says that- “ Energy neither be created nor be destroyed but can be transferred from one form to another”.
When we add heat to a glass of water, due to that heat, the kinetic energy of molecules is increased, which means heat energy is converted to kinetic energy.
What do you think about, how a thermometer works? Correct!!!!!!
The kinetic energy of the water molecules is get transferred to the glass bulb of the thermometer and then to mercury molecules in that glass bulb. And we know that when the kinetic energy of the molecules increases, molecules trying to escape means the substance expands.
And due to the expansion of the mercury, it rises in the capillary and shows a particular reading.
(Do you know mercury is a metal but it is available in a liquid state at atmospheric temperature, and metals are good conductors of heat. That’s why mercury is used in thermometers).
Similarly, your hot cup of tea (at 70 °C) cools down to atmospheric temperature ( about 30 °C) after some time. This means heat is rejected by hot tea or heat is transferred from tea to the atmosphere.
So, heat is converted into a different form of energy or transferred from one point to another point.
Therefore, we can say that
HEAT is a form of energy or HEAT is energy in transit.
And, the temperature shows the direction of heat transfer.
Here, heat is transferred from tea (at 70 °C) to the atmosphere (at 30 °C).
So, we can say that-
HEAT transfers from the body at a higher temperature to a body at a lower temperature.
Example 5: We know that when we rub our hands, we know what happens. Correct, our hands become warm. Here is what happened, our physical energy( muscular energy) is converted to frictional energy ( due to friction between hands), and that frictional energy results production of heat.
Therefore we can say that,
HEAT is a mode of energy transfer.
I think up till now you may have understood about heat, temperature, and its difference.
We will take another example for further clarification.
Example 6: we will take the spherical balls of different materials like brass, copper, iron, silver, etc. of the same size. Then we will heat them up to a certain temperature( say 90 °C).
Then immediately, we will rest them on a paraffin block.
What do you think will happen?
Yes, as I mentioned heat and temperature are different. Heat is dependent upon mass and mass is related to density, and density is different for different materials. So, the heat contained by each spherical ball is different( heat contained is also depends on specific heat and the specific heat of each material is different).
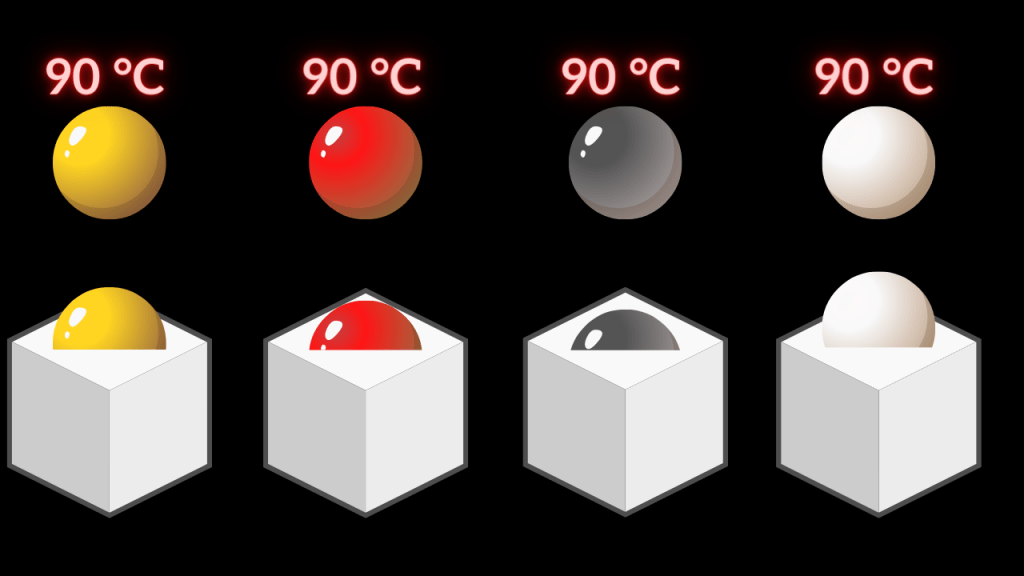 Fig. 3: Experiment to show the different material has a different capacity for storing heat at the same temperature
Fig. 3: Experiment to show the different material has a different capacity for storing heat at the same temperature
Therefore when we rest the spherical metal balls on the paraffin block, each ball penetrates to a different depth. Because heat contained by them is different, although the temperature is the same.
Let’s take another funny example
Example 7: Suppose there is glass filled with 100 Rs. notes and there is another bucket that is filled with 50 Rs. notes. If you have a chance to pick up one, which one will you pick up?
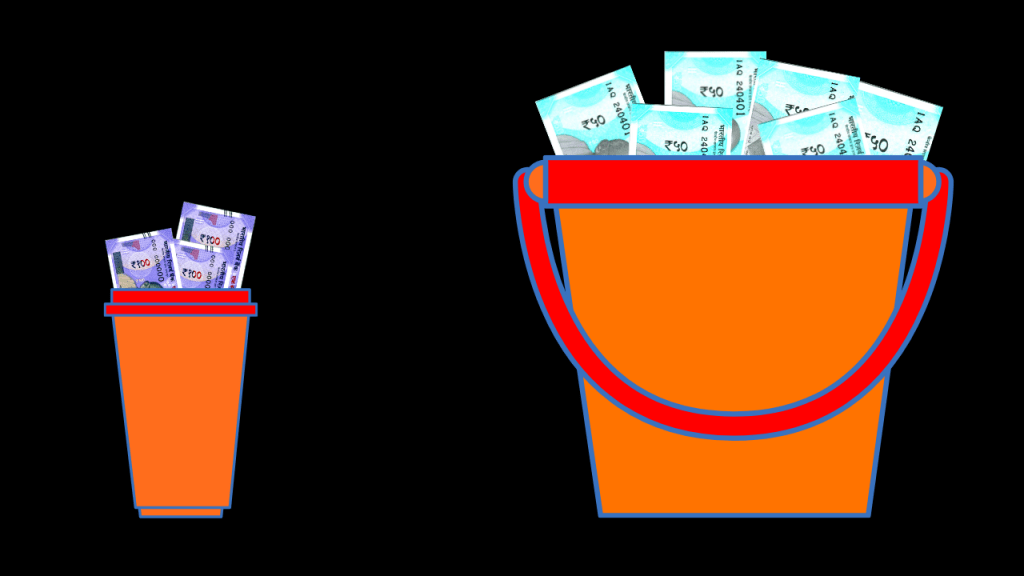 Fig. 4: Real-life example to clarify the difference between Heat and Temperature
Fig. 4: Real-life example to clarify the difference between Heat and Temperature
Exactly, the bucket. Because the value or intensity of each note in the glass is high but bucket has more notes. Means bucket has more money.
Yes, now you have understood. A glass of water has more temperature than a bucket of water, but the bucket has more heat than the glass.
Another amazing example is that in the morning you want warm water to take bath. You have half a bucket of water whose temperature is 30 °C. So, you want hot water to mix with cold water to make it warm.
I will give you a glass of water whose temperature is 80 °C and another half bucket of water whose temperature is 50 °C.
Then which one will you pick up?
Yes, absolutely!
That 50 °C bucket of water, and I think, now no more explanation is needed because you understood the concept.
If you have any queries, please feel free to comment
And share your opinion
Thank you so much if you read up to this line!
The Amazing Fact: Maybe you have noticed in the winter season, suppose there is one piece of metal and one piece of wood on the outside. Both the pieces are in thermal equilibrium with each other (which means both have the same temperature).
But if you touch both of them, you feel metal is much colder than wood.
How is this possible?
Answer: See, as we discussed previously, we feel anything hot or cold because of the temperature difference. Here the temperature of metal and wood is lower than our body temperature. This means heat is flowing from the body (surface of hand) to metal. But the fun fact is that the heat transfer rate is different for wood and metal. Metal is a good conductor of heat than wood. That’s why when we touch metal the heat is more rapidly transferred than the wood. Therefore metal feels much colder than the wood, although both have the same temperature.
Isn’t it amazing !!!😇😉


I must thank you for the efforts youve put in penning this site. I am hoping to check out the same high-grade blog posts by you in the future as well. In fact, your creative writing abilities has motivated me to get my very own blog now 😉
Thank you very much for your feedback. Glad you liked it!!